Relief for your employees
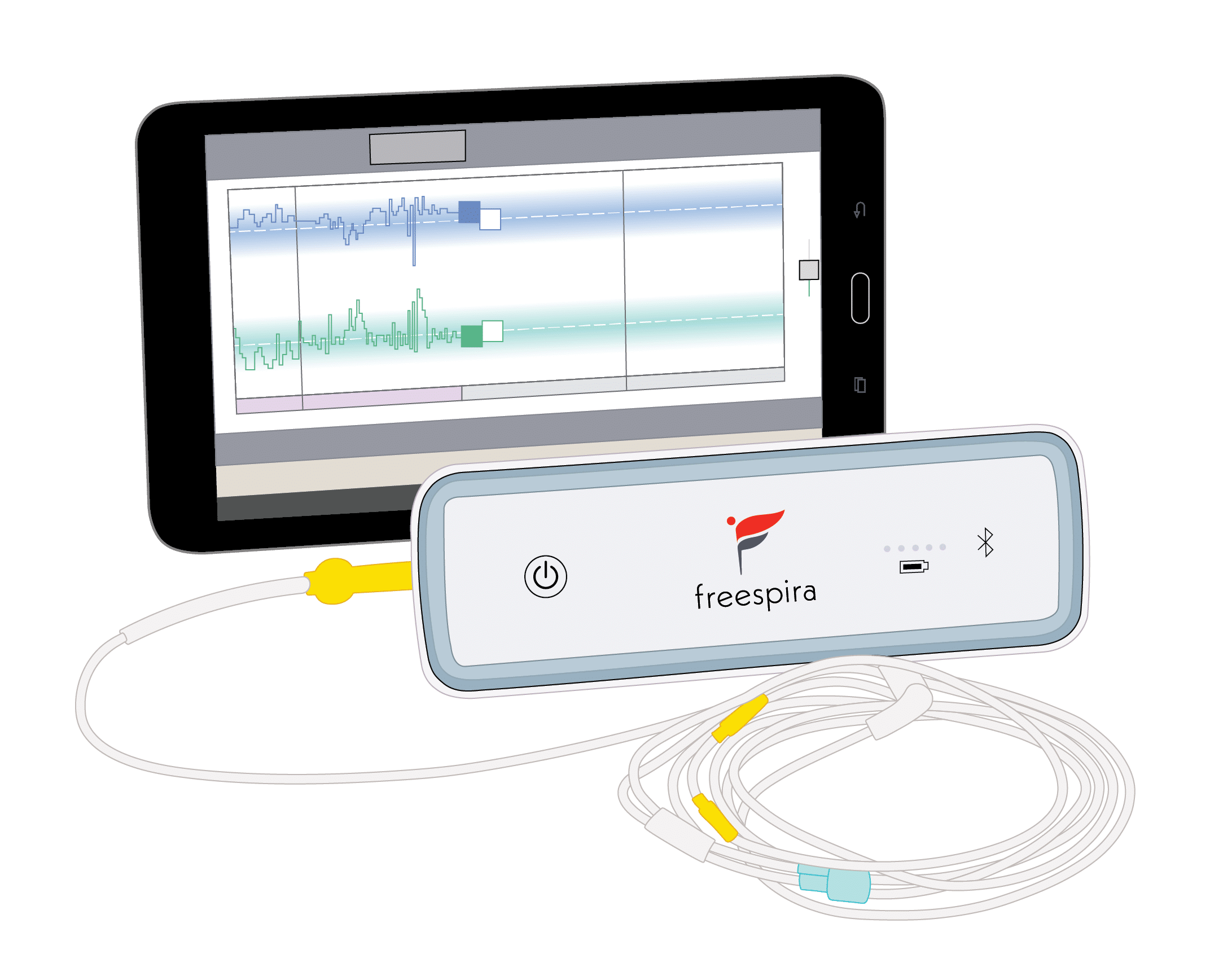
Freespira is the only FDA-cleared treatment proven to reduce or eliminate panic attacks and post-traumatic stress disorder (PTSD) symptoms. Unlike medication or talk therapy, in 28 days Freespira addresses underlying breathing irregularities common in individuals suffering from these debilitating conditions.
HOW FREESPIRA HELPS
Living with anxiety attacks or traumatic stress symptoms can be scary
Typical symptoms may include:
CHEST PAINS
SHORTNESS OF BREATH
UNCOMFORTABLE FLASHBACKS
Freespira is helping to break down access barriers as an at-home treatment option for panic disorder and PTSD that does not require a doctor or therapist visit.
The Results
Practical, proven treatment for anxiety attacks
Panic attacks and PTSD impact plan spend. These conditions lead to significantly higher average additional claims spend per patient:
$0
$0
Panic attacks accompany a range of other health conditions. The 28.3% lifetime prevalence of panic attacks means many people experience a panic attack without meeting the full diagnostic criteria for panic disorder.4 98% of panic disorder patients have at least one behavioral health comorbidity; among the most common, major depressive disorder, anxiety disorder, and alcohol use disorders5
Combined, comorbid prevalence of panic disorder and PTSD adults and teens3
Freespira is easy to use, with high adherence averaging 75%.
Freespira is an accessible at-home treatment that doesn’t require a doctor’s visit.
Freespira is the only FDA-cleared, medication-free digital therapeutic proven to reduce or eliminate panic attacks and PTSD symptoms.
Freespira is proven to lower total medical plan costs by 35%.
"I now notice when my chest tightens from anxiety and then I focus on my breath and the symptoms go away. I feel like I have learned to take control of my anxiety and I will use this awareness for the rest of my life."
—Freespira Patient
IN PEER-REVIEWED STUDIES
Freespira reduced total cost of care by 35%6
Patients experienced symptom reduction:
Health plan saw reduction in medical costs6:
CLINICALLY PROVEN
Get started with accessible mental health support
Freespira is a new, FDA-cleared breathing treatment accessible for all. Getting started doesn’t require a doctor or therapist’s appointment—just a brief clinical assessment to determine suitability.
Typical implementation in 6+ weeks is guided by an onboarding manager and includes clinical team and case manager referral/training, and targeted multi-channel communications to expand membership awareness and engagement.
Freespira is a medication-free, twice daily treatment that normalizes breathing behavior which eliminates or reduces panic and PTSD symptoms. Each Freespira patient receives a sensor and tablet and is assigned a dedicated coach to virtually guide them through treatment sessions. After 28 days, more than 80% of patients saw a significant reduction in symptoms of panic disorder and PTSD.6, 7
Resources for Employees
E-BOOK
Clinical Evidence, Real-World Results
VIDEO
How Freespira
Works
RESEARCH BRIEF
Cost Savings
Study